Diffusion coefficient represents molecular transmission capacity through film because of thermal motion of molecular chain. Diffusion is derived from the mass transfer process of molecular random motion and it is a phenomenon that particles (molecules, atoms) gradually transfer from their original position by micro random motion. In the field of flexible packaging testing, diffusion coefficient is a major parameter for prediction of product quality guarantee period.
1. Diffusion Phenomenon and Fick's Law
From the view of microcosm, diffusion phenomenon in gas has a direct relationship with thermal motion of gas molecule. As shown in Figure 1 the density of composition A (shown using white circle) below the plane S is higher than that above the plane S. During a same time period, because of thermal motion of gas molecule, the number of molecules passing through the plane S upwards are greater than that passing through plane S downwards. Thus there has net mass being transmitted upwards, this cause the diffusion in macroscopic phenomenon.
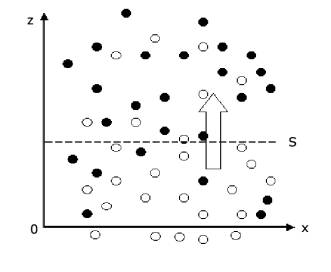
Figure 1. Diffusion phenomenon
The basic formula for description of diffusion phenomenon is Fick's Law :
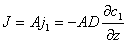
Where: A —— sectional area of diffusion
j1 —— flux per unit area
c1 —— concentration
z —— distance
This formula is one of the expressions of Fick's Law. Fick called “D” in the formula as “a constant depending on property of substance”, which is diffusion coefficient. Fick also derived a general conservation equation in style of Fourier Equation:
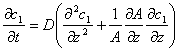
When A stays constant, this conservation equation becomes a basic equation of one-dimensional non-steady diffusion, which is called Fick's Second Law.
2. Influencing Factors of Diffusion Coefficient
As we know, gas molecule needs energy to transfer through film, but the active energy is relative to molecular diameter, so diffusion coefficient decreases with the increase of molecular diameter.
Diffusion is also relative to temperature. With the increase of temperature it is easier for gas molecule to transfer. Diffusion coefficient increases with the increase of temperature. This follows Arrhenius formula:
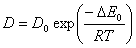
Where: Δ E0 is activation energy of diffusion, it increases with the increase of molecular diameter. The greater the diameter is the harder the molecular diffuses.
3. Diffusion Phenomenon and Applications
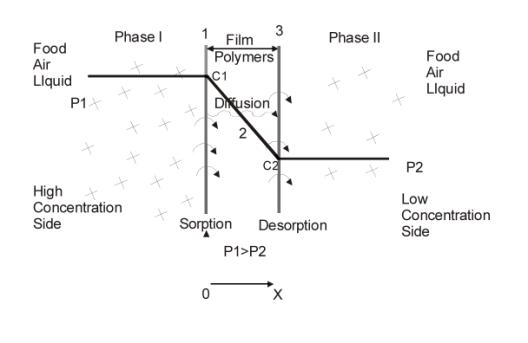
Figure2. diffusion process
From the view of microcosm, permeation process in film is as follows(as shown in Figure 2)
1.Gas molecule hits the surface of film;
2.Solution;
3.Solution balance of gas at high concentration side of film surface;
4.Gas diffuses to the other side of the film because of existence of concentration grads;
5.Desorption
Generally speaking, diffusion is the slowest and the most critical step in permeation process. It has close relation with permeation and solution. When a same kind of gas (such as oxygen) permeates different kinds of films, permeability coefficient is mainly depending on the diffusion coefficient of gas in these films. When different gases permeate a same kind of film, permeability coefficient is mainly depending on the solution coefficient of gas in this film. For a same kind of film, permeability coefficient is proportional to gas transmission rate. According to Fick's Law, we suggest concentration stays the same, if D is very small, gas will need a very long time to permeate through the film, that represents the film has a good performance in macroscopic view; if D is bigger gas will permeate through the film easier, that represents the film has a weaker performance. Depending on specified applications, we can choose suitable diffusion coefficient and other permeability parameter to meet the usages of packaging.
4. How to get diffusion Coefficients
There are many methods to be used to get the diffusion coefficient. Among these methods half-time method, prediction method and time-lag method are usually used. In half-time method, first remove the permeation gas to be tested from inside the polymer, and then make one side of this polymer contact this permeation gas and adjust the equipment into an equal pressure state so that a steady transmission rate will be reached. At last the diffusion coefficient is determined by the time when half of the permeation balance state is reached. The merit of half-time method is high precision, but the test will take a long time and it's difficult to reach a permeation balance state. The prediction method is also called Pasternak numerical method. This method makes the prediction process more convenient due to application of computer science. Comparing the half-time methods, prediction method is faster, but its precision is lower. Prediction method is suitable to be used in diffusion coefficient test of high permeability films.
Time-lag method is one of the common methods used to test diffusion coefficient. In time-lag method, a test is performed under high vacuum. The pressure of test gas at one side equals nearly zero. Diffusion coefficient is calculated by testing the “lag time” before running into balance state.
D = L 2 / 6 θ o
Where:
L is thickness of film. θ o is lag time.
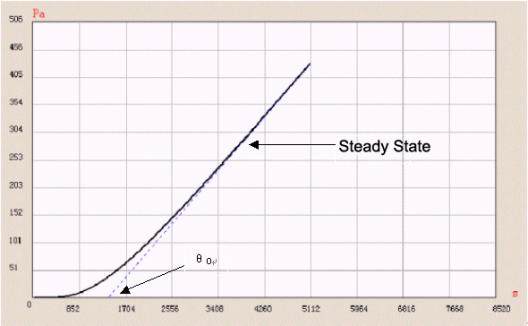
Figure 3 “ Time-lag ” method
Figure 3 shows a tested permeation curve of one sample using Labthink VAC-V1. The whole test process is controlled by computer. Furthermore, VAC-V1 can calculate solubility coefficient and permeability coefficient of a sample, so solubility coeficient can also be got. And by using the fitting function of VAC-V1, we can easily obtain the coincidence relations between diffusion coefficient, solubility coefficient, gas transmission rate and temperatures, so test efficiency is improved and the test becomes easier.
With the increase of application & demand of polymer materials in packaging market and with the fast development of new functional materials, the development and testing of barrier films is not only limited to index such as permeability coefficient and transmission rate, it is more frequently for us to test the diffusion coefficient, solution coefficient etc that affect permeation parameters directly. Thus barrier test of materials is tending toward perfection.
